
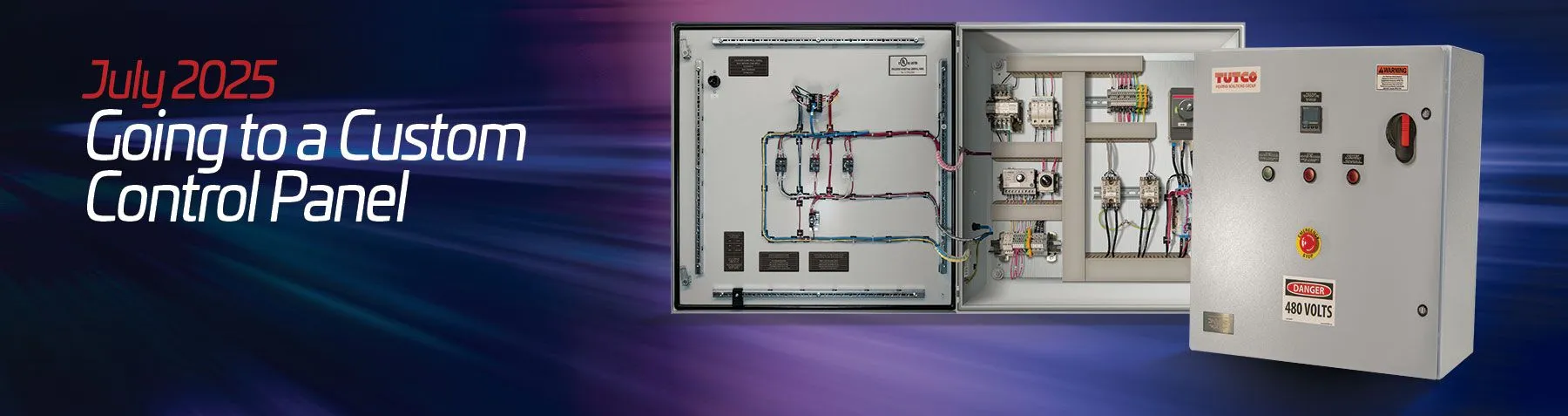
Going to a Custom Control Panel
When people hear the word custom, two things often come to mind: it’s going to be expensive, and it’s going to have a long lead time. Neither of these has to be the case when working with TUTCO Farnam. Designing and manufacturing a custom control panel is about finding the best possible solution for a customer’s specific application. It’s the same approach we take with our process heaters, so it makes sense that we would take the same approach with our control panels.
Customers often are unsure of what they need their control panel to do. They know they need a control panel but they don’t know what they want or need the panel to do. Other customers know exactly what they need and already know that one of our standard panels won’t work for their process. In both cases, we can guide customers through our process—asking the necessary questions to understand the application and the demands of the customer’s system. Through our questions, we often uncover opportunities to control more than just the system’s heater. It may be valves that need to open and close, blowers that require variable speed control, or other pieces of equipment like motors or a linear actuator.
TUTCO Farnam control panels do much more than manage set point temperatures and high temperature limits. We can include controls for motion control, variable frequency drives (VFDs), and programmable logic controllers (PLCs). With a PLC, you can control pretty much any component you want and that you can program them specific to the customer application. We can also incorporate safety features, lighting controls, stack lights, and alarms. We also build controller to manage multi-zone systems. A multi-loop panel doesn’t mean you have to multiply the cost. Once we’ve built the panel and its core components, adding loops or other functions doesn’t necessarily drive up costs in a significant way. The final cost often depends more on the total load and amperage than on how many separate controls are included.
When talking to TUTCO Farnam about a custom control panel, customers do not need to have an understanding of every detail of what they want. They just need to tell us what you want the panel to control and what the desired end results are, and we’ll take it from there. If there are certain brands you prefer, we’re happy to accommodate, but we don’t expect them to have to know what theyu would like to use.
At TUTCO Farnam, we create complete solutions based on our understanding of heating systems and the process they need to work in. Our experience allows us to deliver custom solutions that work at a high level in the applications they are designed for. If you think you need more than a standard control panels and would like to build something tailored to your application, reach out to begin the process.

Understanding the Difference Between Heat and Temperature
by Ian Renwick
In the study of thermodynamics, heat and temperature are two central concepts that govern the behavior of energy within systems. While these terms are often used interchangeably in everyday language, they have distinct scientific meanings that are crucial for accurate interpretation and application in various fields of research and industry. From chemistry to engineering and environmental science, understanding the nuanced differences between heat and temperature is not only essential for proper scientific communication but also for designing efficient processes, conducting experiments, and building models that accurately represent the natural world.
Introduction: Why Distinguishing Heat and Temperature is Essential
In a variety of scientific domains, particularly those involving energy transfer (like our electric heaters), the terms heat and temperature appear frequently. Despite their pervasive usage, they are often misunderstood or used incorrectly in both popular science and professional contexts. Scientists and engineers must be able to differentiate between these terms with precision, as they play distinct yet complementary roles in energy systems.
Heat and temperature are not merely interchangeable descriptors of thermal phenomena. Rather, they represent two entirely different aspects of thermodynamic behavior. Heat is the transfer of energy due to temperature differences, while temperature is a measure of a system's thermal state—the average kinetic energy of the particles within that system. Misunderstanding or oversimplifying the difference between these two can result in inefficient energy systems and potentially dangerous situations.
Technical Definitions: Heat vs. Temperature
To grasp the differences between heat and temperature, we must first define each term in the context of thermodynamics.
Heat: The Transfer of Energy
Heat is the energy transferred between systems or objects due to a difference in their temperatures. This transfer of energy occurs spontaneously from regions of higher temperature to regions of lower temperature, and it continues until thermal equilibrium is reached—meaning both systems or objects have the same temperature. In thermodynamic systems, heat is considered a process—that is, it is not a property of the system itself, but a transfer of energy between systems due to temperature differences. No temperature difference? No heat transfer.
The unit of measurement for heat is the joule (J), though the calorie (cal) is also used in some contexts, especially in food science (in the US). In general, the amount of heat transferred depends on the mass of the substance, its specific heat capacity (a material property that indicates how much heat is required to raise the temperature of a given mass by 1°C, and the temperature change. So where do watts come in? Why all this talk about joules? As it would happen 1 joule per second = 1 watt. So if 600 joules of heat travelled from object A to object B in 30 seconds, that mean there was 20 watts. Watts is the rate of heat transfer. Joules is the amount of heat doing the transferring.
Mathematically, heat transfer can be expressed as:
Q=m∙c∙ΔT where:
• Q is the heat transferred,
• m is the mass of the substance,
• c is the specific heat capacity of the material,
• ΔT is the temperature change.
Here’s where it gets tricky. As that heat is transferring from one place to another, it affects the temperature of where it came from and where it’s going. In other words, the hot bit get cooler and the cold bit gets hotter, until they both reach the same temperature. With that going on, it means that the heat that’s moving from the hot bit to the cold bit is changing, meaning you can calculate how much heat will go from the hot bit to the cold bit in total (from the current condition until they’re both at the same temperature) but not how much heat is transferring at any one instant, because it’s always changing. Well you can, but it’s a trickier calculation.
Temperature: A Measure of Thermal Energy
In contrast, temperature is a measure of the average kinetic energy of the particles in a substance. It reflects the intensity of the heat energy present in a system, not the quantity of heat. Temperature is a property that does not depend on the amount of matter or the size of the system. The higher the temperature, the greater the average kinetic energy of the system’s particles, and that’s it. For example, if you have two bodies of the same material—one large and one small—both at the same temperature, the kinetic energy per particle will be identical. However, the larger body will contain more total energy because it has more particles. You can have a pinhead at 1000°F, or a 10 lbs block of copper at 1000°F.
Temperature is usually measured using instruments such as thermometers, thermocouples, or infrared sensors. The most common units of temperature are kelvin (K), Celsius (°C), and Fahrenheit (°F). The kelvin scale is the standard SI unit, with zero kelvin (0 K) representing absolute zero—the theoretical point at which all particle motion ceases. See an earlier article about different temperature scales if you really want to dig into it (Journey Through Temperature Scales-Fahrenheit, Celsius, Kelvin, Rankine? | Conductive).
Key Conceptual and Practical Differences Between Heat and Temperature
While heat and temperature are related, they differ in several important ways. To clarify these differences, we examine the following distinctions:
1. Extensive vs. Intensive Properties
- Heat is an extensive property, meaning that its value depends on the amount of substance present. The more material there is, the more heat can be transferred or stored. For example, doubling the mass of a substance while keeping its specific heat constant will double the amount of heat required to achieve the same temperature change.
- Temperature, on the other hand, is an intensive property. This means that it remains the same regardless of the quantity of material. Whether you have a liter of water or a pool of water at 25°C, both have the same temperature, but the total heat content will differ based on the mass of the water.
2. Energy Transfer vs. Measurement
- Heat refers specifically to the energy that is transferred from one system to another due to a difference in temperature. It is not stored in a system; rather, it is the energy exchanged during a process. For instance, when a hot metal object is placed in cooler water, the heat from the metal transfers to the water, raising the water’s temperature.
- Temperature is a measure of a system's internal energy state. It is a static property that tells us the degree of thermal motion in the system. When you measure the temperature of a body, you are gauging the average kinetic energy of its constituent particles.
3. Impact on Systems
- Heat transfer has a direct impact on a system’s internal energy, as heat flows in or out of the system. When heat is added to a system, it increases the system’s internal energy, which may appear as a temperature increase or a phase change (called latent heat, when a material melts, freezes, vaporizes (boils), condenses, or goes through sublimation or deposition with no change in temperature). Conversely, removing heat decreases the internal energy of a system.
- Temperature, however, does not transfer energy but reflects the amount of energy present in a system at a given time. It determines the direction in which heat will flow (from high to low temperature) but is not a measure of the energy itself.
Real-World Applications and Implications
Materials Science and Engineering
In materials science, understanding the relationship between heat and temperature is crucial for controlling phase transitions, such as in the annealing or quenching of metals. For example, when cooling a molten metal, the heat must be dissipated efficiently, and the temperature must be controlled to achieve the desired crystalline structure. Similarly, understanding the heat capacity of materials allows engineers to design systems (e.g., engines or heat exchangers) that maintain optimal operating temperatures.
Chemical and Industrial Engineering
In chemical reactions, temperature is a critical factor that influences the rate of reaction and equilibrium position. Catalysis, reaction kinetics, and the thermodynamics of reactions all depend on precise temperature control. On the other hand, the heat produced or absorbed during chemical processes must be carefully managed to ensure the efficiency and safety of the system. Engineers often use heat exchangers to transfer heat from one fluid to another without mixing them, thereby controlling the temperature of various system components.
In our World of Heaters
It’s quite a challenge when we receive a request for a heater with X and Y dimensions and a required temperature of 450°F. It’s impossible to provide a heater like that without knowing a lot more about the system from the materials involved, their mass, their physical geometry, how they’re ‘connected’ to the outside world, what insulation may be present, the time required to reach temperature…. the list goes on. We can provide so many joules per second (or watts, were you paying attention?), but not a specific temperature without knowing everything about the application. Even then, theory is only theory. It never perfectly reflects the real world.
That’s where temperature controllers come into play and really save the day. You need 450°F? How about we provide you a heater that gets really really hot and you measure its temperature with a thermocouple (or something else) and control it with a temperature controller. Then you can get your 450°F all day long. That’s what a lot of people do.
Conclusion: The Significance of Understanding Heat and Temperature
While heat and temperature are interrelated, they represent two distinct concepts in thermodynamics. Heat is the transfer of energy between systems due to temperature differences, while temperature is a measure of the average kinetic energy of the particles in a substance. These distinctions are not merely academic but have profound implications for practical applications in a wide range of scientific fields. A thorough understanding of both concepts allows scientists, engineers, and researchers to design more efficient systems, improve energy management, and make more accurate predictions in fields ranging from materials science and engineering to climate science and environmental studies. By properly distinguishing between heat and temperature, professionals can ensure that their work is grounded in solid thermodynamic principles and can avoid the common pitfalls that arise from mixing these two essential concepts.
The Future is Bright for the TUTCO Heating Solutions Group!
by Jeff Elrod
 If you have been reading this article for the past few months, you know that TUTCO has never been content with where it is. We have changed our core markets on more than one occasion along with diversifying our offering, especially in the last decade. Our company has always tried to be on the cutting edge of electric heating technologies which is where the name Heating Solutions Group came from. We are not changing that approach now. If anything, we are becoming more aggressive in offering solutions for a changing world.
If you have been reading this article for the past few months, you know that TUTCO has never been content with where it is. We have changed our core markets on more than one occasion along with diversifying our offering, especially in the last decade. Our company has always tried to be on the cutting edge of electric heating technologies which is where the name Heating Solutions Group came from. We are not changing that approach now. If anything, we are becoming more aggressive in offering solutions for a changing world.
This approach starts out with our engineering and design teams. While some may say they have decades of heat experience, we can say that we have centuries of experience in design and innovation with our engineering and R&D teams in all our locations in North America and the world. That, along with our parent company willing to invest in our innovation and growth, even created an internal team to focus on internal innovations in all our divisions. We are not only evaluating new designs but also improving our processes to allow us to stay competitive in existing markets.
We are not just changing in the technical side but also looking to improve our business as a whole. We are not looking for customers but rather looking for partners. We want to be part of your team. We want to be your partner involved in your whole project, not just providing heat but providing a heat solution that is exactly what you need, not just an off-the-shelf solution you can get by with.
Heat is being used in many more applications than ever before and electric heat is becoming more prominent in industries that have historically used other methods. We have seen a rise in food service opportunities with multiple divisions picking up new customers in this changing market. We see huge opportunities in the decarbonization industry with so much innovation and progress being made by so many companies. These markets include pollution mitigation, emissions solutions, regulatory compliance, thermal energy storage, energy creation and land fill waste reduction. Data centers are becoming part of business systems around the world more than ever and we are here to provide them with the electric heat solutions needed for these buildings and the equipment in them from decarbonization, load banks and more.
Providing complete heat solutions is going to be a big part of this growth. We not only want to provide the heaters, but a complete package including all the controls and devices required for the process. With industrial control panels being a market we are entering, we can even offer control panel solutions for non-heat opportunities.
We are ready to kick in that work ethic that has brought us to where we are from a resistor company to the electric heater solutions provider we are today, not just in the appliance and HVAC world but in different processes from about every major industry and many smaller ones. I am not sure what lies in our future, but I am excited to find out.

FEATURE INDUSTRY
Food Service Solutions
TUTCO heat's up the food service industry
In foodservice applications, precision matters. From the perfect roast on a small-batch coffee blend to the seal on a package of fresh produce, consistent and reliable heat is essential. TUTCO serves the needs of the industry with a range of process air heaters flexible heaters, and conductive solutions.
With decades of experience designing and manufacturing electric heating elements, TUTCO is a trusted partner for original equipment manufacturers (OEMs) throughout the food and beverage industry. Whether customers are building something brand new or refining an existing design, our team brings a deep understanding of thermal solutions to the table—solutions that power everything from convection ovens and shrink wrappers to warming cabinets and steam tables.
Restaurants and food processors are looking to move away from fossil fuels through electrification. TUTCO is helping these forward-thinking manufacturers embrace this shift, offering cleaner, more efficient heating technologies that align with today’s sustainability goals. With TUTCO’s proven electric heat solutions, companies are cutting their carbon footprint without sacrificing performance.
TUTCO’s heaters are built for the full range of foodservice applications—cooking, prep, packaging, and serving. We've designed heaters for moisture removal, plastic sealing, coffee roasting, and more. Our experience spans everything from the kitchen floor to the production line. Chances are, if you need a heater for your application, we’ve already made one like it. And if not, we’ll custom-build a solution that meets your specific needs.
FEATURE VIDEO
TUTCO SureHeat's Over Temperature Protection
At TUTCO SureHeat, we understand the critical importance of delivering custom heating solutions that perform reliably and include robust failsafe protection to prevent unscheduled downtime—even under the most severe operational errors. As part of that commitment, TUTCO SureHeat engineers developed the ultimate protection for resistive wire heating: our patented Over Temperature Protection (OTP). By integrating the heater’s Type "K" thermocouple sensors with external limit controls, OTP safeguards heating elements from premature failure caused by limited or no airflow.
At TUTCO SureHeat, we deliver heating systems that are not only highly reliable but also inherently safe. That’s exactly why we developed our patented Over‑Temperature Protection (OTP)—an integrated safeguard designed specifically for resistive wire heating elements.
How OTP Works
Multi-point temperature sensing: Built into every heater are Type K thermocouples—strategically located on the element, at inlet and outlet points—to continuously monitor temperatures across critical zones.
External limit control: These thermocouple signals feed into an independent OTP limit circuit housed in our control cabinet. Working alongside PID-based power control, this dedicated safety circuit cuts off heater power immediately if predefined thresholds are exceeded.


